Intro to Thermochemistry Pt 2: Calorimetry and Enthalpy
oh boy, calorimetry! this is where things become a bit math-y, but i'd say it's not too bad at this point! good luck!
u might recognize the word "calorie" in "calorimetry".
 |
| we need calories! |
that's a very smart observation of u! calories r just a measure of energy - the amount of heat used to raise the temperature of a gram of water by one degree Celsius. this "calorie" is different from what u might hear nutrition people talking about though. those weirdos r talking about kilocalories, also written as Calories. 1 Cal = 1000 cal. a little confusing right?
anyway, this concept of using a specific amount of heat to raise a gram of something 1°C is gonna be pretty important for calorimetry...........
but what IS calorimetry anyway?!?!?!?
 |
| some pretty high tech stuff! |
Measuring Energy Changes - Calorimetry
i've mentioned bomb calorimeters in the last section, but what exactly are they used for? it's right in the name:
calorimetry - the technological process of measuring energy changes in a system
we know that during reactions, heat energy gets transferred - but now we need to talk about exactly how much energy was transferred! sadly, it's not always enough just to say "ummmmm.... i think a lot of energy was transferred"...
this is where calorimetry comes in!
calorimetry - the technological process of measuring energy changes in a system
we know that during reactions, heat energy gets transferred - but now we need to talk about exactly how much energy was transferred! sadly, it's not always enough just to say "ummmmm.... i think a lot of energy was transferred"...
this is where calorimetry comes in!
 |
| here's a pic of a bomb calorimeter! realllyyyy expensive so people be using styrofoam cups. nice |
calorimetry relies on careful measurements of masses and temperature changes.
the first calorimetric formula we'll learn combines the following three factors to represent the quantity of heat (q) transferred in an energy change:
the first calorimetric formula we'll learn combines the following three factors to represent the quantity of heat (q) transferred in an energy change:
- mass (m), the quantity of matter that exists in the system - measured in grams (g)
- specific heat capacity of the substance (c); the amount of heat needed to raise the temperature of a unit mass (e.g. gram, kilogram) of a substance by 1°C - usually measured in J/(g°C)
- change in temperature (ΔT); the difference between the temperature before and after the change has happened - measured in °C
- remember that Δ means "change/difference in", so ΔT = T2 - T1
q = mcΔT
this equation means that the amount of heat transferred during a reaction can be found by relating the mass, type of substance, and temperature!
(NOTE: quantities of heat are calculated as absolute values, so we always use a positive ΔT value!
in my calculations, i show this by putting |these vertical bars| on either side of the operation - this means "absolute value"!)
(NOTE: quantities of heat are calculated as absolute values, so we always use a positive ΔT value!
in my calculations, i show this by putting |these vertical bars| on either side of the operation - this means "absolute value"!)
sample problem: how much heat would be transferred if 100.0 g of water was cooled from 25.0°C to 10.0°C?
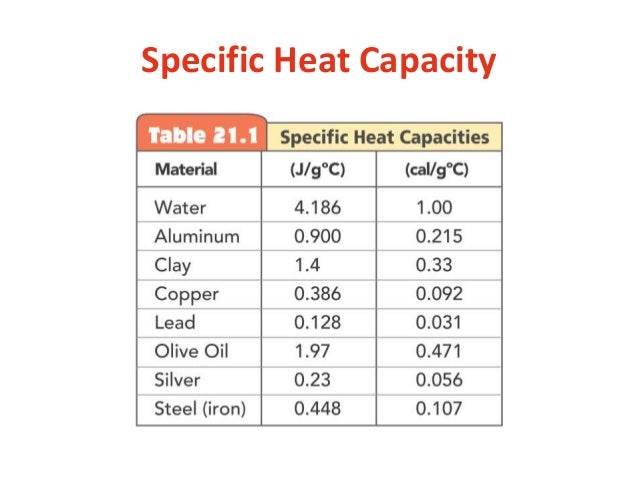
always start by listing what you're given and asked for! i like to do this so i don't have to keep re-reading the question for info - if you're not great at reading, this is especially important XD! specific heat capacity will always be given in a data table
 | |
|
q = ???
mass (m) = 100.0 g
initial temperature (T1) = 10.0°C
final temperature (T2) = 25.0°C
temperature change (ΔT) = |T2 - T1|
temperature change (ΔT) = |10.0°C - 25.0°C|
temperature change (ΔT) = |-15.0°C|
temperature change (ΔT) = 15.0°C
specific heat capacity (c) = 4.18 J/(g°C) (given from the table)
mass (m) = 100.0 g
initial temperature (T1) = 10.0°C
final temperature (T2) = 25.0°C
temperature change (ΔT) = |T2 - T1|
temperature change (ΔT) = |10.0°C - 25.0°C|
temperature change (ΔT) = |-15.0°C|
temperature change (ΔT) = 15.0°C
specific heat capacity (c) = 4.18 J/(g°C) (given from the table)
now that we have all the info we need, we can sub these values into our equation and then solve for q!
a couple of notes for doing math in chemistry:
- be sure to always write out what formula you're using - if you're trying to prove a case in court and they don't know how you got your calculations... that sucks
- keep the units in your calculations!!! the math you do to your numbers will also apply to your units. if u treat them as u would treat variables, they should cancel to give you the right units in your final answer
q = mcΔT
q = (100.0 g)(4.18 J/(g°C))(15°C)
q = (100.0 g)(4.18 J/(g°C))(15°C)
q = 6270 J
congrats! our final answer is 6270 joules, also written as 6.270 kilojoules, or also written as 6.270 kilojuuls! XD just kidding juuls are bad for you! XD
 |
| we're gonna be using lots of water glass examples |
Heat Transfer and Enthalpy Change
so we know what heat transfer is: the transfer of kinetic energy between particles of substances. cool and simple! but... ummmmm..... what's enthalpy??????
enthalpy (H) is the total energy within a system. this can come from...
- kinetic energies of all the moving parts in a system - electrons, atoms, bonds, molecules, etc
- potential energy stored in bonds and nuclear particles
right now there's no way to directly measure this, so instead we measure the change in enthalpy (ΔH) that occurs during a reaction!
remember the "law of conservation of energy"
- the total energy of a system and its surroundings is fixed, regardless of how the energy is transferred or converted

- the potential energy in the system is converted to kinetic energy
- the kinetic energy (i.e. heat) is transferred to the surroundings
- the change in potential energy (ΔH) of the system equals the change in kinetic energy (q) of the surroundings! neato!
This can be summarized in the following relationship, which means that the energy change in a system is the same as the amount of energy that was transferred with its surroundings:
ΔH system = ±|q surroundings|
Types of Enthalpy Changes
enthalpy changes can accompany any type of change in matter. the only difference is in the magnitudes of these changes
physical changes:
- the energy to overcome intermolecular forces
- involve a relatively small ΔH (ranges from 1 to 100 kJ/mol)
 |
| ice melting is an example of a physical change |
chemical changes
- the energy to overcome chemical bonds
- involve a relatively large ΔH (ranges from 100 to 10 000 kJ/mol)
 |
- the energy to overcome nuclear forces
- involves HUGE ΔH (ranges from 10^10 to 10^12 kJ/mol)
- (10 000 000 000 to 1 000 000 000 000 kJ/mol)
 |
| this is why nuclear power generators became a big thing - they're clean (relative to fossil fuels) and produce a lot more energy than combustion! |
that's all for today folks! thanks for tuning in!
omg this is so educational i never knew how to do math before!
ReplyDelete